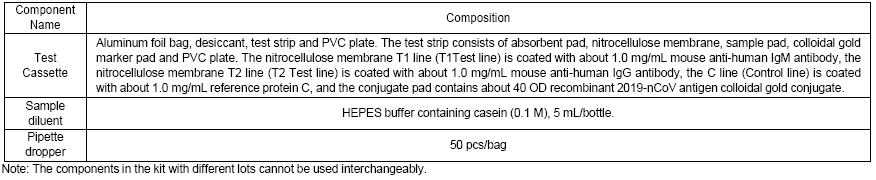
【Principle】
The detection kit uses the principle of solid-phase immunochromatography: the separation of components in a blood sample through a medium by capillary force. 2019- nCoV specific IgM and IgG antibodies in blood samples bind to colloidal gold-labeled viral antigen. Each cassette is a dry medium that has been coated with a recombinant 2019-nCoV antigen (conjugate pad), mouse anti-human IgM antibody (“T1” test line) , mouse anti-human IgG antibody (“T1” test line) as well as a reference protein (“C” control line) (Figure 1). Once the diluted serum/plasma/ whole blood /peripheral blood is loaded into the sample well, it diffuses upward via capillary force. When it passes through the conjugate pads, the colloidal gold-labeled 2019-nCoV antigens will bind to 2019-nCoV IgM and IgG, forming colloidal gold-labeled antigen- IgM and/or IgG complexes. The complexes continue to flow along the nitrocellulose membrane by capillary force. If 2019 nCoV IgM antibody is present in the sample,
the T1 test line will be bound by the mIgM-IgM- colloidal gold-labeled antigen complex thereby generating red color (red T1 line). If there is no 2019 nCoV IgM antibody present in the sample, no color is generated. The unbound immune complexes and antibodies continue to flow to the T2 test line, where mouse anti-human IgG antibody binds with 2019 nCoV IgG antibody to form mIgG-IgG-colloidal gold-labeled antigen complex and generating red color (red T2 line). The unbound colloidal gold conjugates continue to flow and are bound by the line C (Control line), indicating the reaction is completed.
【Product Performance]】
1.Lowest limit of detection
Test with the in-house LOD references. S1 and S2 are positive for novel coronavirus IgG antibody, negative for IgM antibody; S3 is negative for novel coronavirus IgG/IgM antibodies; S4 and S5 are positive for novel coronavirus IgM antibody, negative for IgG antibody; and S6 is negative for novel coronavirus IgG/IgM antibodies.
2.Negative coincidence rate
Test with the in-house negative references, and the results are all negative for novel coronavirus IgG/IgM antibodies, with a coincidence rate of 100%.
3.Positive coincidence rate
Test with the in-house positive references. PC01-PC05 are all positive for novel coronavirus IgG/IgM antibodies, with a coincidence of 100%; PC06-PC10 are all negative for novel coronavirus IgG antibody, and all positive for IgM antibody, with a coincidence rate of 100%; PC11-PC15 are all negative for novel coronavirus IgM antibody, and all positive for IgG antibody, with a coincidence rate of 100%.
4.Precision
Intra-batch difference: Test with the in-house repetitive references. CV1 and CV2 are positive for novel coronavirus IgG antibody and negative for IgM antibody; CV3 and CV4 are negative for novel coronavirus IgG antibody and positive for IgM antibody, with uniform color development.
Inter-batch difference: Test with the in-house repetitive references. The results of the kit of three batch numbers are shown as follows: CV1 and CV2 are positive for novel coronavirus IgG antibody and negative for IgM antibody; CV3 and CV4 are negative for novel coronavirus IgG antibody and positive for IgM antibody, with uniform color development.
5.Analytical specificity:
5.1 Cross reaction
This product will not cross react with positive samples of human coronavirus HKU1, OC43, 229E, influenza A H1N1 virus, seasonal H1N1 influenza virus, H3N2, H5N1, H7N9, influenza B Yamagata, Victoria, respiratory syncytial virus, parainfluenza virus, rhinovirus species A, B and C, adenovirus types 1, 2, 3, 4, 5, 7 and 55, coxsackievirus (enterovirus species B), enterovirus 71 (enterovirus species A), enterovirus 68 (EV-D68) (enterovirus species D), EB virus, measles virus, human cytomegalovirus, rotavirus, norovirus, mumps virus, varicella-zoster virus, mycoplasma pneumoniae, chlamydia pneumoniae IgG/IgM antibodies.
5.2 Interferents
When bilirubin ≤0.2 g/L, triglyceride ≤10 g/L, hemoglobin ≤5 g/L, rheumatoid factor ≤500 IU/mL, antinuclear antibody titer ≤1:240, anti-mitochondrial antibody titer ≤1:160, HAMA ≤20 ng/mL, total IgG ≤50 mg/L and total IgM ≤5 mg/L, they will not interfere with the test results. Oseltamivir, levofloxacin, ceftriaxone, zanamivir, interferon alpha (IFN-α), ribavirin, peramivir, lopinavir, ritonavir, arbidol, azithromycin, meropenem, tobramycin, histamine hydrochloride, phenylephrine, oxymetazoline, sodium chloride, beclomethasone, dexamethasone, flunisolide, triamcinolone acetonide, budesonide, mometasone and fluticasone have no effect on the test results.
6.Hook effect
Hook effect will occur at the concentration levels that exceed the lowest limit of detection of IgG antibody of this product by more than 1280 times and the lowest limit of detection of IgM antibody by more than 640 times. If novel coronavirus pneumonia is highly suspected but the antibody test result is negative, the sample should be re-tested after dilution.
7.After the specific IgM positive sample is destroyed, the IgM antibody test result is negative, and the IgG antibody test is not affected.
8.Heparin sodium and EDTA anticoagulants have no effect on the detection of this kit.
9.The precision test is conducted by different test personnel at different time with this kit, and the results comply with the requirements of product performance.
10.For virus infection samples from different regions, the lowest limit of detection and detection repeatability of the reagent comply with the requirements.
11.Clinical study
The clinical trial of this product was carried out in 5 sites based on the criteria for disease confirmation/exclusion specified in the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia. The enrolled cases were suspected cases of novel coronavirus infection, including 201 confirmed cases and 369 excluded cases, with 51 early cases in confirmed cases. Clinical sensitivity of this product: 91.54% (95% CI: 86.87%, 94.65%) and specificity: 97.02% (95% CI: 94.74%, 98.33%). The sample types for clinical evaluation were serum and plasma. After preliminary evaluation, it is basically confirmed that the clinical performance of the product can meet the emergency needs of the epidemic. The clinical data for the product after marketing will be further collected to confirm the clinical performance of the product.
【Precautions】
1. This product is for in vitro diagnosis only, and cannot be reused.
2. Professionally trained operators are required to carry out the test. Before using the kit, please read the instructions carefully and perform the test in accordance.
3. It is essential to ensure that test laboratories adhere to appropriate biosafety practices.
4. National guidelines on the laboratory biosafety should be followed in all circumstances.
5. The desiccant in the aluminum foil bag shall not be taken internally.
【Sample Requirements】
1. The assay is suitable for human serum/plasma/whole blood.
2. Sediments or suspensions may affect results. Remove by centrifugation at 3,000g for 10 min before testing.
3. Hemolyzed, or hyperlipidemic blood samples should not be tested.
4. Collect whole blood/plasma samples using a heparin sodium or EDTA anticoagulant blood collecting tube. Samples should be run on the same day as collection. If not, whole blood samples can be stored at 2-8°C for 3 days, and serum/plasma samples can be stored at 2-8°C for 7 days, or at -20 °C or lower for 12 months. Avoid repeated freezing and thawing of samples.
5. Bring the sample to room temperature (18°C-28°C) before processing. Frozen samples should be completely thawed, and mixed well before testing. Avoid repeated freezing and thawing.
【Instruction】
Please read instructions carefully before use.
1. Bring the Test cassette, sample diluent, and sample to room temperature before testing. Conduct test at room temperature.
2. Take out the test cassette from the aluminum foil bag and place it on a flat and dry surface.
3. Add one drop of sample to the sample well of the cassette using a pipette dropper, followed by adding 3 drops of diluent into the same well after opening the cap of the diluent bottle.
4. The results can be interpreted after 10 minutes.
5. After the test, the used test cassette, and pipette dropper shall be discarded as biomedical waste.
【Interpretation of Results】
The test results are be interpreted as the followings:
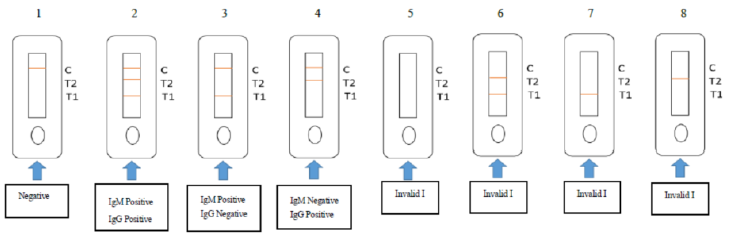
1. Negative for 2019-nCoV: The test line (T1 and T2) does not appear, but the quality control line (C) is colored
2. IgM Positive and IgG Positive for 2019-nCoV: All lines including two Test lines (T1 and T2) and Control line (C) are colored
3. IgM Positive and IgG Negative for 2019-nCoV: Both the T1 test line and Control line (C) are colored, while T2 test line does not appear.
4. IgM Negative and IgG Positive for 2019-nCoV: Both the T2 test line and Control line (C) are colored, while T1 test line does not appear.
5-8. Invalid: There is no colored Control line (C) appears. The results are invalid regardless of whether colored lines appear on both of the test lines (T1and T2) or any of the test lines (T1 or T2); Repeated testing is required.